Natural Health Products (NHPs), like vitamins, supplements, and probiotics, are widely used in Canada and are a key focus in regulatory affairs training. To improve consumer safety and clarity, Health Canada has introduced new NHP labelling regulations. These changes aim to make product information easier to read and understand.
If you’re in regulatory affairs or studying for a pharma career, it’s important to grasp these updates. This blog outlines what’s new, why it matters, and how it affects compliance and public trust.
Why These Updates Matter
Natural Health Products include everything from multivitamins to herbal supplements and probiotics. As demand for these products grows, so too does the responsibility to ensure that consumers are informed and protected. Health Canada’s new labelling requirements aim to eliminate ambiguity, improve clarity, and align NHP standards with those of non-prescription drugs.
But these updates aren’t just about compliance. They also reflect broader concerns around public health, transparency, and market accountability, making them a critical area of focus for today’s regulatory professionals. Here are the changes:
A More Informative Product Facts Table
The biggest structural change is the introduction of a Product Facts Table, a standardized layout that lists vital product information in a familiar, easy-to-navigate format. Consumers can now find key details like uses, directions, active ingredients, and warnings at a glance.
This change makes it simpler for users to compare products and assess risks before they buy, reducing misuse and improving safety.
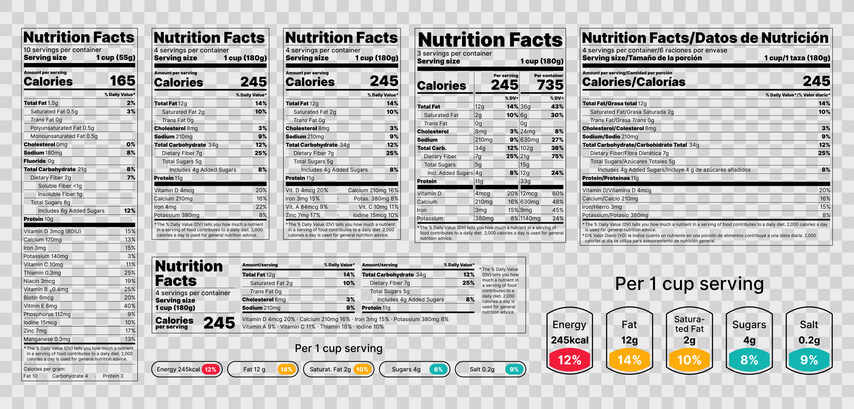
As explored in our regulatory affairs courses, the addition of a product facts table is the biggest update.
Clearer Language for Better Understanding
Gone are the days of scientific jargon. Health Canada now requires plain language across all NHP labels. That means health claims, dosage instructions, and risk statements must be written in terms the average consumer can understand. This shift promotes clarity and reduces the chances of misinterpretation, especially for consumers with limited health literacy.
Enhanced Font Standards
Font size and legibility are no longer optional considerations. Labels must now meet minimum font size requirements, ensuring that critical information is easy to read. This is particularly important for vulnerable groups like seniors or people with vision impairments.
Transparency Around Allergens
New rules mandate full allergen disclosure. Common allergens like gluten, soy, or tree nuts must be clearly listed to help prevent adverse reactions. These rules bring NHPs closer in line with food labelling standards and give consumers peace of mind when selecting products.
Mandatory Expiry Dates and Lot Numbers
As you’ll learn in regulatory affairs training, Health Canada is placing greater emphasis on traceability. Expiry dates and lot numbers must now be prominent and easy to locate. These identifiers are essential in managing recalls and confirming product safety, particularly for sensitive items like herbal treatments and nutritional supplements.

As learned in regulatory affairs training, Health Canada is emphasizing traceability.
Standardized Risk Messaging
Risk information, including warnings about side effects, drug interactions, and safety instructions, must now follow a standard format and wording. This ensures consistency across brands and helps consumers better understand potential risks.
Required Contact Information
All labels must include contact information for the product license holder. Whether it’s an email, phone number, or website, this provides a direct link for consumer inquiries or reporting issues, further reinforcing industry accountability.
What About Implementation?
Under the most recent update (March 2025), all natural health products (NHPs), regardless of when their product licence is issued, must comply with the new labelling regulations by June 22, 2028.
Originally, products licensed on or after June 21, 2025, were expected to meet the new labelling requirements immediately. However, Health Canada has since introduced a Ministerial Exemption Order to ease the rollout. Under this exemption:
- Existing NHPs (licensed before June 21, 2025) have a transition period until June 22, 2028, to update their labels.
- New NHPs (licensed between June 21, 2025, and June 21, 2028) are temporarily exempt from the new labelling rules and may continue using the previous labelling format, provided they meet all other regulatory requirements.
By June 22, 2028, all NHPs on the market must comply with the new labelling requirements, and the exemption will be lifted. This phased approach gives manufacturers time to make the necessary changes while allowing regulatory affairs professionals to manage the transition in a practical and compliant manner.

Our regulatory affairs training offers regulatory affairs courses tailored to real-world needs.
What This Means for Regulatory Affairs Training Grads
For professionals or those training to enter the industry, these new labelling rules offer an opportunity to demonstrate leadership. From updating documentation to overseeing packaging compliance, skilled regulatory personnel will play a critical role in ensuring smooth adoption.
Suppose you’re looking to step into or advance within this space. In that case, our regulatory affairs training offers regulatory affairs courses tailored to real-world needs in compliance, safety, and regulation, designed to help you thrive in your career.
Are you interested in pursuing a regulatory affairs career?
Contact AAPS for more information.




