LET OUR EXPERTS HELP YOU GET LICENSED.
AAPS Consulting will help you navigate the Health Canada licensing process from start to finish.
INQUIRE TODAY
Thank you for contacting AAPS consulting.
A representative from our team will be in touch with you shortly.

WHY CHOOSE AAPS
Our Services Guaranteed AAPS Consulting was created to help business owners like you navigate the heavily-regulated and often complicated Canadian cannabis industry and leverage our experience working with Health Canada to meet regulatory compliance.
We offer full support, guiding you through the application process with the sole objective of positioning your business for approval and success.
Your Business – Our Expertise. Since the advent of the MMPR in 2014, AAPS has developed a proven track record of helping industry leaders obtain licenses and maintain compliance. No matter what regulatory challenges arise, we have the expertise to help you navigate the regulatory hurdles.
OUR CLIENTS









OUR CONSULTING SERVICES
AAPS Consulting provides full support and materials required to become a Licensed Producer with proven results from dozens of successful applications submitted to Health Canada. We can help you develop a best-in-class Quality Management System that is customized to your operations and will meet Health Canada compliance requirements. AAPS consulting can assist with facility design, build-out requirements and establish security protocols and procedures. We provide customized GPP, GMP & GACP compliant SOPs for Production, Processing and Quality Assurance and can recommend vendors and prospective partners for build-out, security, design, equipment, and seed-to-sale tracking.

CULTIVATION AND PROCESSING LICENCE APPLICATION
AAPS Consulting can assist you with the Health Canada applications for cultivation, processing and nursery licenses.
Whether you are applying as a stand-alone or combining multiple licence classes, the process is complex and demanding. We will help you implement a strategic approach to your application, from security and personnel requirements to Good Production Practices (GPP), optimizing your facility design and process flow, identifying potential gaps, navigating the Cannabis Tracking and Licencing System (CTLS) and submitting a professional site evidence package.

MEDICAL SALES LICENCE APPLICATION
Medical Sales licence applicants are required to meet the rigorous physical security measures established by Health Canada and submit a detailed Organizational Security Plan. You will also need a robust record keeping system in order to closely track patient registration, verification of Health Care practitioners and product quantity shipment checks. Our team of experts will perform a gap analysis to ensure your system and processes are compliant in order to successfully obtain a sales with possession licence.

RESEARCH AND ANALYTICAL TESTING LICENCE APPLICATION
Utilized primarily by educational and non-profit institutions, the R&D licence allows for a wide variety of activities that includes sensory testing, formulation, practical training and method development.
An Analytical Testing Licence is required for all laboratories conducting the required third-party testing for pesticides, microbials, and heavy metals as well as cannabinoid and terpene content. Our highly experienced team with decades of experience in analytical laboratory operations and can assist with all aspects of your application.

RETAIL LICENCE APPLICATION AND STAFF TRAINING
AAPS Consulting has assisted with multiple cannabis retail licence applications across Canada. We provide a number of different services including application support, inventory management strategies, menu curation and more. We also assist with hiring and training, providing customizable workshops and programs to ensure staff are informed on the regulations and possess in-depth product knowledgeso they can communicate effectively with customers.

ADDITIONAL ACTIVITIES AND AMENDMENTS
AAPS consulting provides post-licensing support in the form of site audits, inspections, second opinions and expert advice. Our team can help you apply for import or export permits, EU-GMP Certification, sales and provincial wholesale listings as well as add or change key personnel, expand your operations and inform Health Canada of any administrative changes.

QUALITY ASSURANCE SERVICES
To achieve regulatory compliance and preserve product safety, identity, strength, purity and quality, AAPS Consulting will help you implement a robust and modern Quality Management System within your facility. From SOPs, seed-to-sale tracking integration, document control and inventory management we provide you with customized solutions that take into consideration your operations, industry best-practices, as well as Health Canada regulation and compliance requirements.
Our services also include developing the Preventive Control Plan (PCP) and HACCP for cannabis edibles processing.
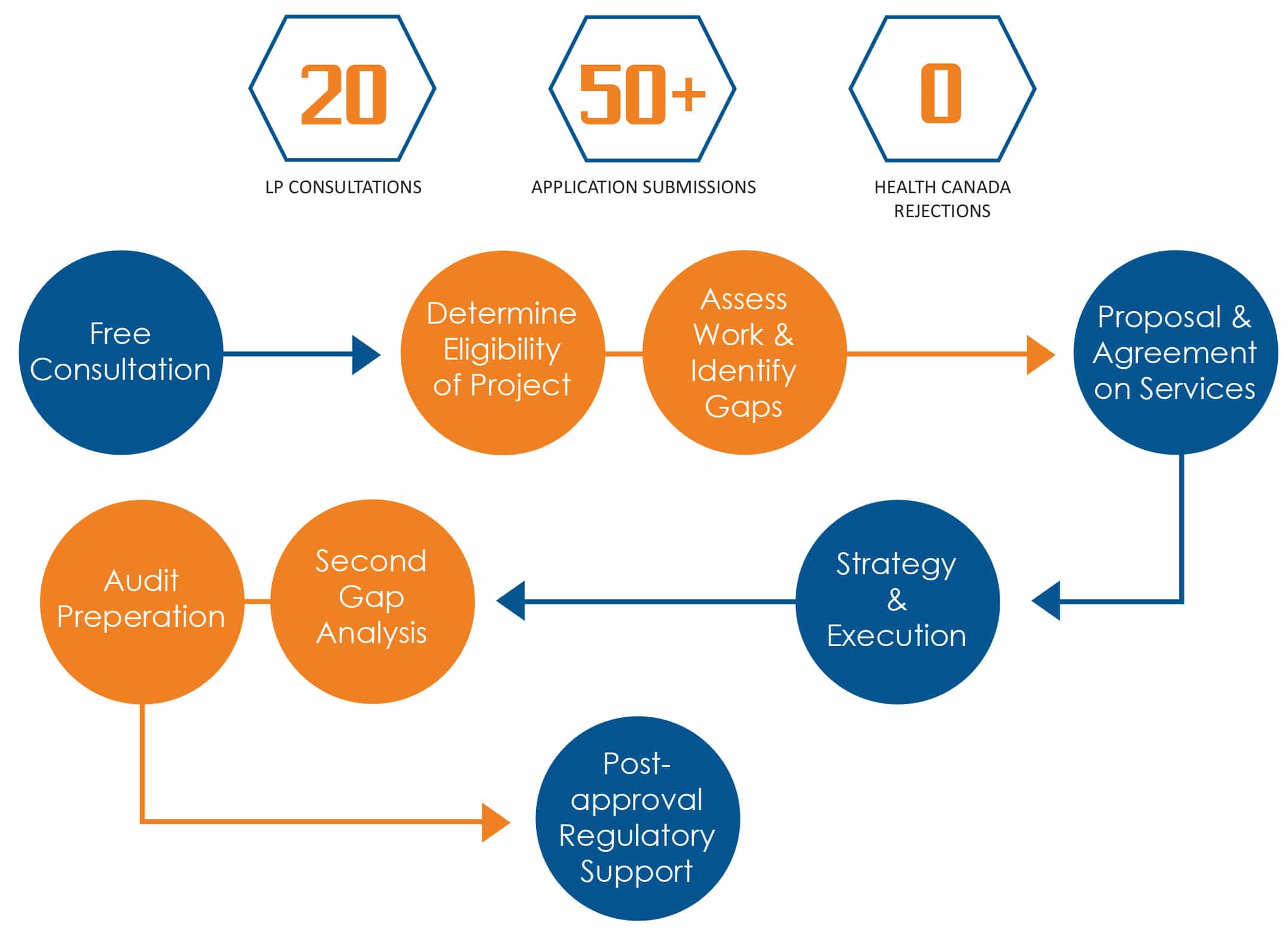
OUR PROCESS

Application
Compliance
Reports
Document
Submission
Review
Evidence
Package
Full Application
for all classes

Regulatory
Good
Manufacturing
Practices
Good Production
Practices (GPP)
Mock Audit
Preparation
Record Keeping

Design
Customized SOPs
Floorplan
Material & People
Flow Review
Security
(Organizational
Security Plan)

Recruitment
Executive
Search
& Staffing
On-Site and
Virtual Training
Professional
Development
Recognized Certification
WANT TO LEARN MORE ABOUT HOW WE CAN HELP?
*By clicking on the button, your browser will open/download “Cannabis Consulting Brochure” in PDF format.


